Stem Cell Therapy for Type 2 Diabetes in Malaysia: Evidence-Based Guide 2025
Estimated Reading Time: 7-8 minutes
Table of Contents
Introduction
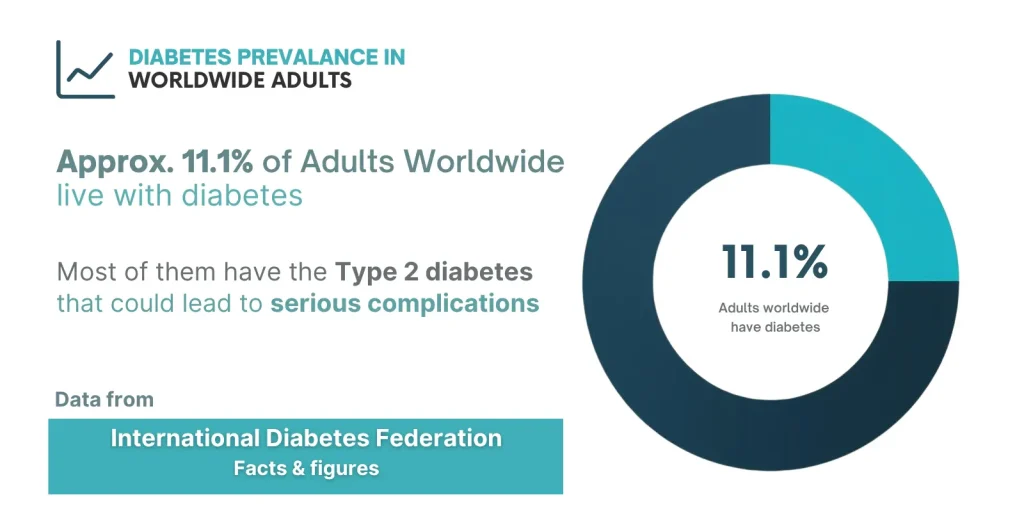
Type 2 diabetes (T2DM) is now a global epidemic. According to the International Diabetes Federation, about 11.1 % of adults worldwide (aged 20-79) currently live with diabetes, and over 90 % of those cases are type 2. In many Asian countries, the prevalence is rising steadily due to urbanization, sedentary lifestyles, and dietary changes.
This metabolic disorder affects blood sugar control and can lead to serious complications including heart disease, kidney failure, nerve damage, and vision problems, according to the World Health Organization (WHO).
While conventional treatments—oral medications, insulin therapy, and lifestyle modifications—help manage blood sugar levels, they primarily address symptoms rather than the underlying causes. This has led to growing interest in stem cell therapy for type 2 diabetes in Malaysia as a potential regenerative approach. However, it remains under clinical evaluation in Malaysia and is not considered a standard treatment.
This guide provides evidence-based information about stem cell therapy for Type 2 diabetes: how it works, what current research shows, treatment options in Malaysia, costs, and important considerations for patients.
Understanding Type 2 Diabetes
Type 2 diabetes is a chronic metabolic condition characterized by two main problems:
1. Insulin Resistance: The body’s cells become less responsive to insulin, preventing glucose from entering cells efficiently. This causes blood sugar to accumulate in the bloodstream.
2. Pancreatic Beta Cell Dysfunction: Over time, insulin-producing beta cells in the pancreas become damaged or exhausted, reducing the body’s ability to produce adequate insulin.
Common Risk Factors
- Obesity and excess abdominal fat
- Sedentary lifestyle
- Family history of diabetes
- Age (risk increases after 45)
- Unhealthy diet
- Ethnic background (Southeast Asians have higher risk)
Current Treatment Limitations
Standard treatments include oral medications (metformin, SGLT2 inhibitors), injectable medications (GLP-1 agonists, insulin), lifestyle modifications, and regular monitoring.
Key Limitation: These treatments manage symptoms and slow disease progression but do not repair damaged pancreatic cells or fully reverse insulin resistance. Many patients require increasing medication doses over time, and some eventually need insulin injections despite initially managing with oral medications alone.
How Stem Cell Therapy Works for Type 2 Diabetes

What Are Mesenchymal Stem Cells (MSCs)?
For Type 2 diabetes treatment, doctors primarily use mesenchymal stem cells (MSCs)—adult stem cells with the ability to differentiate into various cell types, secrete growth factors, modulate immune responses, and promote tissue repair.
MSCs can be obtained from:
- Umbilical cord tissue (Wharton’s jelly from donated cords)
- Bone marrow (usually from the pelvic bone)
- Adipose tissue (fat, via liposuction)
Mechanisms of Action
When administered to patients with T2DM, MSCs may work through several mechanisms:
1. Pancreatic Beta Cell Regeneration: MSCs may support the regeneration of damaged pancreatic beta cells primarily through paracrine signaling, and in some cases, may also differentiate into insulin-producing cells, potentially enhancing natural insulin production.
2. Improved Insulin Sensitivity: MSCs release factors that may help cells respond better to insulin, addressing one of the core problems in Type 2 diabetes.
3. Anti-Inflammatory Effects: Chronic inflammation contributes to insulin resistance and beta cell damage. MSCs have demonstrated anti-inflammatory properties.
4. Protection of Existing Cells: MSCs may protect remaining healthy beta cells from further damage caused by high blood sugar, oxidative stress, and inflammation.
It’s important to understand that stem cell therapy for type 2 diabetes in Malaysia is currently classified as an adjunctive or experimental treatment, not a replacement for standard diabetes care.
Note: Stem cell therapy is considered experimental and should complement, not replace, standard medical care.
Clinical Evidence: What Research Shows
Multiple clinical trials have investigated MSC therapy for Type 2 diabetes:
Safety Profile
A 2023 review published in Frontiers in Endocrinology analyzed six clinical trials involving 262 patients with Type 2 diabetes and found that mesenchymal stem cell (MSC) therapy was generally safe.
Key Safety Observations
- Overall Safety: No serious adverse events were reported across all six trials.
- Mild and Temporary Side Effects Noted: Nausea, Mild fever, Localized discomfort at the injection site
- No major long-term safety issues have been identified so far, although data beyond two years remain limited.
Efficacy Findings
A 2020 meta-analysis in Journal of Diabetes Investigation reviewed 9 clinical trials involving 227 patients and reported the following outcomes:
- HbA1c Reduction: MSC therapy was associated with a statistically significant decrease in HbA1c, indicating improved long-term blood sugar control.
- Variable C-Peptide Response: Some patients showed increased C-peptide levels, suggesting enhanced endogenous insulin production, but the response was inconsistent across studies.
- Insulin Requirement Changes: A subset of patients experienced reduced insulin or medication needs, though this benefit was not universal.
- Inter-Patient Variability: The degree of improvement varied considerably among individuals, likely due to differences in disease stage, baseline beta-cell function, and therapy protocols.
Clinical Outcomes
Some studies have reported:
- Reduction in HbA1c levels (a measure of long-term blood sugar control)
- Decreased fasting blood glucose
- Improved C-peptide levels (indicating better insulin production)
- Reduced insulin requirements in some patients
Important Limitations
- Most studies involve relatively small patient numbers
- Follow-up periods typically limited to 12-24 months
- Multiple clinical trials registered on ClinicalTrials.gov continue investigating optimal protocols
- Results vary significantly between individuals
- Optimal protocols not yet standardized
- Long-term effects beyond two years not well established
Note: Research into stem cell therapy is more advanced for Type 2 diabetes compared to Type 1. Type 1 diabetes involves autoimmune destruction of beta cells, making treatment more complex, and remains largely experimental.
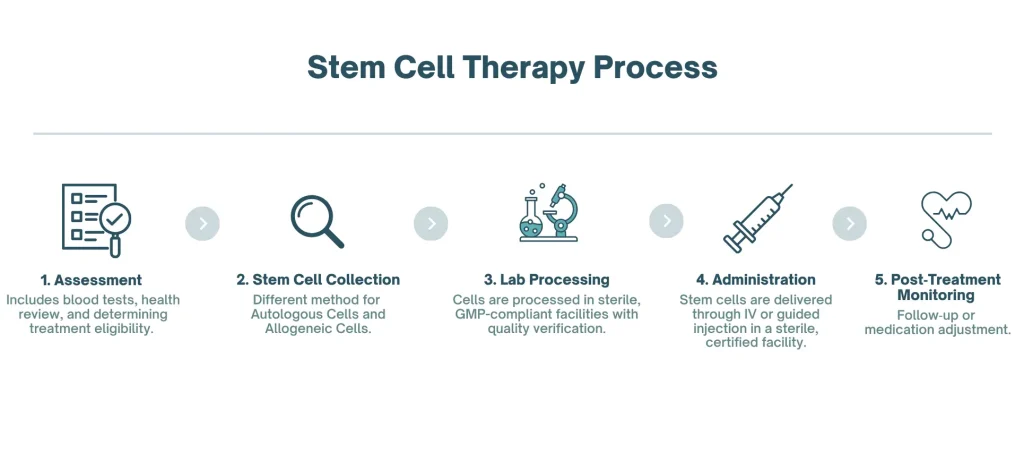
The Treatment Process
Pre-Treatment Assessment
Before stem cell therapy, patients undergo:
- Comprehensive medical history review
- Physical examination
- HbA1c, fasting glucose, and C-peptide testing
- Kidney and liver function tests
- Complete blood count
- Discussion of expectations and potential outcomes
Ideal candidates typically have Type 2 diabetes that is not well-controlled despite medications, have adequate remaining beta cell function, and maintain realistic expectations.
Stem Cell Collection and Administration
If Using Autologous Cells (Patient’s Own)
- Bone marrow extraction: Local anesthesia, needle inserted into pelvic bone, 30-60 minutes
- Adipose tissue harvest: Mini-liposuction under local anesthesia, 45-90 minutes
If Using Allogeneic Cells (Donor)
- Umbilical cord-derived MSCs from certified tissue banks
- Pre-processed and quality-tested
- Ready for immediate use
Administration
MSCs are typically administered through clinically approved methods, and the specific approach may vary depending on the facility and the patient’s condition. The cells are processed in sterile, GMP-compliant facilities and undergo quality verification.
Post-Treatment Care
Immediate (1-2 weeks)
- Continue regular diabetes medications as prescribed
- Monitor blood sugar levels closely
- Rest and avoid strenuous activity
Medium-Term (1-6 months)
- Regular follow-up appointments
- Repeated blood tests to monitor changes
- Medication adjustments as needed under doctor supervision
Long-Term (6+ months)
- Continued monitoring of HbA1c and other markers
- Assessment of treatment effectiveness
- Discussion of potential repeat treatments if beneficial
Most patients do not see immediate results. Effects, if they occur, typically become apparent over 3-6 months.
Availability in Malaysia
Stem cell therapy for Type 2 diabetes is available in Malaysia through select private medical facilities. It is not currently offered as standard care in public hospitals.
Regulatory Framework
Stem cell therapy in Malaysia is regulated under:
- Cell and Gene Therapy Products (CGTP) Guidelines – National Pharmaceutical Regulatory Agency (NPRA)
- Private Healthcare Facilities and Services Act 1998
- MOH Guidelines on Stem Cell Research and Application
What This Means: Facilities must comply with safety standards and maintain proper licensing. However, the experimental nature means it is not classified as standard medical care, long-term efficacy is still being studied, and insurance coverage is typically not available.
Choosing a Treatment Facility
Verify
- Valid MOH and NPRA licensing
- Qualified specialists in endocrinology or regenerative medicine
- GMP-certified laboratory facilities
- Transparent treatment protocols
- Thorough informed consent process
- Comprehensive post-treatment monitoring
Warning Signs to Avoid
- Facilities promising guaranteed cure or diabetes reversal
- Lack of medical professional oversight
- Unclear about cell sources and processing
- No proper informed consent process
Cost and Financial Considerations
Estimated Range: RM 60,000 to RM 100,000 (approximately USD 13,000-30,000)
The price variation depends on:
- Cell source: Autologous bone marrow, adipose, or allogeneic cord (costs vary by provider and dosage)
- Facility type: Clinic vs. hospital
- Treatment protocol: Single vs. multiple administrations
- Additional services: Imaging, follow-up, and monitoring
Insurance Coverage
Most Malaysian health insurance policies do not cover stem cell therapy for diabetes as it is considered experimental. Patients typically pay out-of-pocket. Some facilities offer payment plans.
Cost-Benefit Considerations: While upfront costs are high, some patients consider potential long-term savings from reduced medication expenses and fewer diabetes-related complications. However, these benefits are not guaranteed and vary significantly.
Benefits, Risks, and Limitations
Potential Benefits
Based on research and clinical experience, some patients may experience:
Short-Term (3-6 months)
- Modest reduction in HbA1c levels
- Improved fasting blood glucose
- Better blood sugar stability
- Potential reduction in medication requirements
Medium-Term (6-18 months)
- Continued improvement in glycemic control
- Enhanced insulin sensitivity
- Better energy levels
Long-Term Considerations
- May help slow progression of complications
- Could delay transition to insulin therapy in some patients
- Effects may diminish over time, requiring repeat treatments
Important Note: Individual responses vary widely. Some patients experience significant benefits, while others notice minimal or no improvement.
Risks and Side Effects
Common (Mild and Temporary)
- Discomfort at injection or extraction sites
- Mild fever or flu-like symptoms for 1-2 days
- Temporary fatigue
- Nausea
Rare but Possible
- Infection at injection site (less than 1%)
- Allergic reaction to processing solutions
- Blood sugar fluctuations requiring medication adjustment
- No therapeutic response (approximately 20-40% of patients reported in some trials and clinical studies)
Important Limitations
Clinical Limitations
- Not a cure for diabetes
- Cannot reverse severe pancreatic damage
- Does not eliminate need for blood sugar monitoring
- Requires continued lifestyle management
- May not be effective in advanced cases
- Standard medications typically must be continued
Scientific Limitations
- Optimal protocols not yet standardized
- Long-term safety data beyond 3-5 years limited
- Mechanisms not fully understood
- Predictors of response not well-established
Practical Limitations
- High cost without insurance coverage
- Requires multiple medical visits
- Not available in all regions
- May need repeat treatments
- Results take months to become apparent
Who Should Avoid This Treatment
Stem cell therapy may not be appropriate for:
- Active infections or fever
- Recent cancer diagnosis or treatment (within 5 years)
- Severe kidney disease
- Uncontrolled cardiovascular disease
- Certain blood disorders
- Pregnancy or breastfeeding
- Active autoimmune conditions
- Unrealistic expectations of cure
Frequently Asked Questions (FAQ)
Q1: Is stem cell therapy safe for diabetes?
Clinical trials involving over 260 patients have shown MSC therapy is generally safe, with only minor side effects like temporary nausea or injection site discomfort. However, long-term safety beyond 3-5 years requires more research.
Q2: How effective is stem cell therapy for Type 2 diabetes?
Results vary significantly between individuals. Some studies show improvements in HbA1c levels and reduced medication needs, while others show minimal effects. Approximately 60-70% of patients may experience some degree of benefit based on preliminary data.
Q3: Can stem cell therapy cure diabetes?
No, stem cell therapy is not a cure for diabetes. It may help improve blood sugar control and pancreatic function in some patients, but it does not eliminate the disease. Continued diabetes management is necessary.
Q4: How long does it take to see results?
Most patients who respond to treatment begin noticing improvements 3-6 months after administration. Effects may continue to develop over 6-12 months. Immediate results should not be expected.
Q5: How long do the benefits last?
Based on current research, benefits when they occur may last 12-24 months. Some patients maintain improvements longer, while others may need repeat treatments.
Q6: Will I be able to stop my diabetes medications?
Most patients cannot completely stop medications. Some may be able to reduce doses under careful medical supervision, but this varies by individual and should only be done with doctor guidance.
Q7: Is it covered by insurance in Malaysia?
Generally not. Most Malaysian health insurance policies classify stem cell therapy as experimental and do not provide coverage. Patients typically pay out-of-pocket.
Conclusion
Stem cell therapy for type 2 diabetes in Malaysia represents an emerging treatment option that may benefit certain patients as part of comprehensive diabetes management. Current research shows potential for improving blood sugar control and pancreatic function through regenerative mechanisms, though outcomes vary significantly.
Key Takeaways
- Stem cell therapy is not a cure but may help improve glycemic control in some patients
- Treatment is experimental and not considered standard medical care
- Safety profile appears favorable based on current studies
- Costs range from RM60,000-100,000 without insurance coverage
- Individual responses vary; approximately 60-70% may see some benefit
- Results take 3-6 months to appear and may require repeat treatments
- Should be viewed as complementary to, not replacement for, proven diabetes care
Before Pursuing Treatment
Patients considering stem cell therapy should:
- Consult qualified endocrinologists or diabetes specialists
- Verify clinic credentials and regulatory compliance
- Understand the experimental nature and uncertain outcomes
- Review detailed cost information
- Maintain realistic expectations
- Continue with proven diabetes management strategies
- Ensure proper follow-up care will be provided
For more information about diabetes treatment options or to discuss whether stem cell therapy might be appropriate for your situation, consult with a qualified healthcare provider or contact us.
ℹ Disclaimer
This article is for informational purposes only and does not constitute medical advice. Stem cell therapy for Type 2 diabetes is an experimental treatment with ongoing research into its safety and effectiveness. Individual results vary significantly. Always consult licensed healthcare professionals for personalized medical advice and treatment decisions. Availability and regulations of stem cell therapy may vary by country.
Last Updated: October 2025